A recent study published in the journal Science paves the way for treating severe brain diseases previously considered incurable. Researchers from the Broad Institute at MIT and Harvard have developed a new gene delivery system that overcomes one of the main challenges of gene therapy in the brain: crossing the blood-brain barrier.
Blood-brain barrier? How is this possible?
The blood-brain barrier, a highly selective membrane that protects the brain from toxins and pathogens, prevents most drugs and gene therapies from reaching their destination. This has limited the development of effective treatments for neurological diseases such as Alzheimer’s, Parkinson’s, and Huntington’s for years, but the new study is promising for neurological diseases.
You may be wondering what findings so far have been promising; this is due to a new system called BI-hTFR1, which is nothing more than a gene delivery system using an adeno-associated virus (AAV) modified to bind to a human protein called the transferrin receptor. This protein is abundant in the blood-brain barrier, allowing the AAV to transport its genetic content into the brain efficiently. This becomes highly relevant because this protein in the blood-brain barrier protects the brain from toxins and pathogens. All this is made possible because BI-hTFR1 uses an AAV as its base. AAVs are natural viruses that infect human cells but do not cause disease. They are considered safe and effective for gene therapy, so in this way, BI-hTFR1 carries a therapeutic gene, which can be used to correct a genetic mutation that causes a brain disease or to produce a protein that can help treat the disease.
How is the team conducting the study, and what are the findings?
The research is currently being carried out in the laboratory of Ben Deverman, a scientist at the institute and senior director of vector engineering at the Broad. The company responsible for the study is Apertura Gene Therapy. For this study, they are using mice, using the application of AVV (Adeno-Associated Virus) injected into the animal’s bloodstream, from which the scientists realized that the mice could express humanized transferrin receptors.
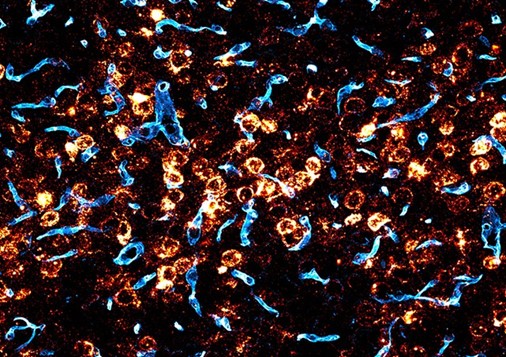
This finding, to date, has demonstrated a result in the brain with more significant potential than that currently approved by the FDA, which would be AAV9. The team found that the blood-brain barrier was crossed 40-50 times more often than AAV9. In addition to this finding, they discovered that the new AAV reached up to 71% of neurons and 92% of astrocytes in different brain regions, neurons, and astrocytes being essential cells for information processing and the general functioning of the nervous system. Thus, if BI-hTFR1 can be used in humans, it could help correct genetic mutations, produce therapeutic proteins, and even modulate brain activity, which regulates neurotransmitters and can act on seizures in neurological diseases.
Deverman states, “If this AAV does what we think it will do in humans, based on our studies with mice, it will be much more effective than the current options.” The scientists are hopeful that the AAV can be replicated in humans.
And what has changed so far?
Dr. Ben Deverman’s team in Denver sought to understand what other researchers were doing so that they could act differently. Previously, researchers tested AAVs on animals to find the best ones for gene therapy. They created many different versions of AAVs and tested them on animals to see which ones worked best. The researchers didn’t know precisely how the AAVs reached the cells they needed to treat.
This made it challenging to use AAVs in humans. If scientists didn’t know if an AAV would work in humans or how it worked, ensuring it was safe and effective was challenging. The Denver team currently uses a human protein: The new method uses an AAV that binds to a protein that already exists in the human body. This means that the AAV is more likely to work in humans.
To do this, they start by screening a library of AAVs in a test tube for those that bind to a specific human protein that they hope will be effective. After this, they began testing on mice
that are considered promising for treatment and have also gone through the process of expressing the protein; the team used cells and mice that have been modified to express the human transferrin receptor. This made it possible to assess the ability of AAVs to bind to and enter brain cells, and from this screening, they could find the BI-hTFR1 AAV.
BI-hTFR1 is still under development, but the preliminary results are promising. With further research and clinical trials, this innovative technology could revolutionize the treatment of neurological diseases, offering hope to millions of patients worldwide. In addition, BI-hTFR1’s versatility in carrying a wide range of genes opens doors for treating various neurological diseases, from diseases caused by genetic mutations to neurodegenerative diseases. This includes diseases such as Gaucher’s, Rett’s,
Huntington’s, Parkinson’s, Alzheimer’s, and epilepsy are currently extremely delicate diseases to live with, both from the point of view of the patients and the family and friends who accompany them.
In medicine, the search for new treatments and cures is a constant journey full of exciting discoveries and, at times, uncertainties. The study of BI-hTFR1 in mice represents one of those moments where curiosity is intertwined with hope. Initially, innovative results can arouse a mixture of fascination and apprehension. It’s natural to question this new compound’s applicability and long-term effects. However, it is crucial to remember that science is built on questioning and rigorous investigation.
For more on Health, visit Mecella.


No responses yet